Project Portfolio

New Bioprocessing Facility

Microbial Fermentation & Protein Purification Pilot Plant

Beigene
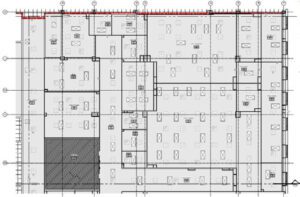
Gene Therapy Manufacturing Facility
The Gene Therapy Manufacturing (GTM) facility is a new, 200,000SF two-story facility for viral vector cGMP manufacturing. Phase 1 of the project includes three processing suites for adherent or suspended AAV manufacturing served by a common solution preparation area. The overall project will be BSL2-capable and has a goal of LEED Platinum.

Global Standardization Projects
A global CDMO in pharmaceutical manufacturing providing process development and cGMP production in cell culture, microbial fermentation and gene therapies. Texas site will become the largest single-use CDMO campus in North America.

New Bioprocessing Facility
Design assist and construction of a new manufacturing bioprocessing facility for a leading CMO. With a tight schedule, modular components were on the project site within 4 weeks of the start of the project.
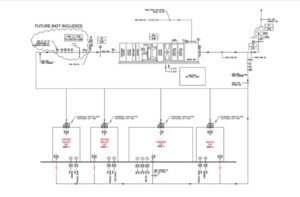
cGMP Manufacturing Facility
The 23,000 SF facility will focus on gene therapy CDMO process development and have Good Manufacturing Practice (GMP) manufacturing capabilities. The areas in this facility will include Process and Analytical Development Labs, GMP Manufacturing Clean Rooms and Supporting Areas, QC Labs, Warehouse, Office Space, New Storage Mezzanine above Warehouse and a New Office Mezzanine.

Beigene
Beigene, a global biotechnology company focused on the development of cancer treatment drugs, brought CleanSpace on board to construct 4,200 SF of clean space for sterility testing, sampling, and weigh/dispense rooms.

Confidential Client US Government Mid-West
Fast-track design and installation of entire freezer and building for COVID-19 vaccine storage for Novavax. Project was completed as part of Operation Warp Speed.
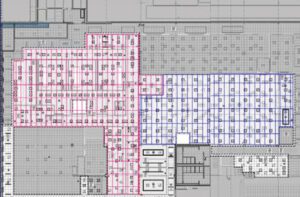
PCT Filling Operation
CleanSpace is providing the architectural envelope for the cleanroom spaces associated with Moderna’s Personal Cancer Therapy sterile filling operation.

New Manufacturing Facility
Design/build/commissioning of a new manufacturing facility of single-use products to be used in gene therapy and bioprocess manufacturing facilities. Project included the master planning of the 210,000 sf facility, and the design of the fit-out of this facility in three phases. The development phases are developed concurrent with ongoing manufacturing operations.

Microbial Fermentation & Protein Purification Pilot Plant
Construction of a new cGMP flexible clinical process suite to support early clinical manufacturing of novel process technologies. This 1,000 SF suite will be developed within Metagenomi’s existing building in Emeryville, CA.

