Cleanroom Industry Insights

Good Design Practices for Gene Therapy CleanSpaces

CleanSpace Manufacturing

CleanSpace Welcomes Jenni McCarthy as VP of Business Development

What is a BSL Cleanroom?
The Biological Safety Level (BSL) serves as a system of biocontainment designation, essential for safeguarding personnel against potential pathogenic exposure in research and manufacturing environments. This system outlines specific requirements for protecting those working in these settings. It’s crucial to note the revision of the Biological Safety Level (BSL) standards in the Biosafety in Microbiological and Biomedical Laboratories (BMBL) in 2020. This update brought about the 6th edition of these standards and included several key updates.

How to Clean Your Cleanroom
Maintaining a pristine environment is paramount in scientific research, semiconductor manufacturing, and additional, highly regulated industries. Enter the cleanroom: a controlled space meticulously engineered to minimize airborne particles and contaminants. But how do we keep these critical spaces sparkling clean? This blog will offer a contemporary perspective on the tasks supporting cleanroom cleaning and explore best practices, frequencies, and regulations.

GMP & Modular Cleanrooms
In this blog post, we are going to discuss the importance of not only adhering to Current Good Manufacturing Practices (GMP) but also safeguarding the integrity of every process and product with proper personnel flows, process flows, and material flows when building modular cleanrooms. Let’s look at the history of GMP, cleanrooms, and how the practices intertwine, each driven by the need for better control and efficiency in specific industries.

Sustainability and Reduced Environmental Impact of Modular Cleanrooms
Once upon a time, building a Cleanroom was an environmental nightmare. Traditional “stick-built” methods relied heavily on resource-intensive materials like brick, mortar, and concrete, leaving a trail of extraction and emissions in their wake. On-site construction generated mountains of debris, often exceeding 30% of the total materials used, and the energy guzzled to power tools, transport materials, and maintain operations painted a bleak picture for the planet.
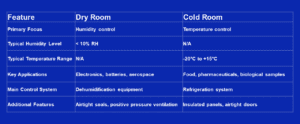
Cleanrooms, Cold Rooms, & Dry Rooms
In this post, we are looking into the world of controlled environments to discover the intricacies of two distinct yet vital spaces – dry and cold rooms. These controlled environments play crucial roles in various industries, from scientific research to manufacturing and pharmaceuticals. So, if you’ve ever wondered about the differences, applications, and principles governing their operations, you’re in the right place.

What are ATMPs?
The term Advanced Therapy Medicinal Products (ATMPs) officially emerged in 2007, with the adoption of Regulation (EC) No 1394/2007 by the European Commission. This regulation established a legal framework for these innovative therapies in the European Union.
However, the concept and development of ATMPs have a more extended history, tracing back to earlier forms of cell and gene therapies that emerged in the 1980s and 1990s. These earlier therapies, often called gene therapy, somatic cell therapy, or tissue-engineered therapies, faced regulatory challenges due to their novelty and complexity.
The introduction of the ATMP terminology in 2007 represented a significant step forward by recognizing these therapies as a distinct category with specific regulatory requirements. This categorization facilitated their development and approval by providing a clear framework for manufacturers and regulators.
A CleanSpace Guide to Cleanroom Classifications
If you want to build a cleanspace, there’s a critical choice from the start – modular or standard construction. While both options serve the same purpose of maintaining pristine conditions, they differ in their approach and advantages. In this article, we’ll dive into the fascinating realm of cleanroom classifications and explore the unique qualities that set modular cleanrooms apart from their traditional counterparts.
Cleanroom classifications constitute a vital framework within the field of controlled environments, serving as a standardized system for categorizing and defining the cleanliness and contamination control levels of cleanrooms.
These classifications are essential for ensuring product quality, safety, and regulatory compliance across various industries, including pharmaceuticals, electronics manufacturing, aerospace, and healthcare. Here is a comprehensive overview of cleanroom classifications:

CleanSpace Welcomes Jenni McCarthy as VP of Business Development
CleanSpace has welcomed Jennifer McCarthy as the new Vice President of Business Development. Jenni boasts a robust 24-year tenure in commercial construction and design, with an emphasis on the life sciences sector.

5 Innovative Applications of Modular Cleanrooms in the Cell & Gene Industry
Modular cleanrooms significantly expedite the deployment of new therapies in the cell and gene therapy industry. Their prefabricated nature allows for rapid assembly and implementation, a crucial advantage in a field where time is of the essence. This rapid deployment directly translates into a shorter time to market for new therapies, thus facilitating timely access to innovative treatments. Moreover, the inherent flexibility of modular cleanrooms supports swift adaptation to evolving research needs and production scales, ensuring that the development and scaling up of new therapies can progress without significant delays.

